- Cancer:
Cancer can be though of as a breakdown of the regulation
of cell division, apoptosis, differentiation, and other
essential functions.
- Origin
of Cancers: Cancers develop from changes that
occur to a single cell and its consequential
proliferation (clonality). The resulting proliferation
of cells is called a malignant tumor. (A benign tumor
is also a proliferation of cells, but it does not
invade neighboring tissues or spread to other parts of
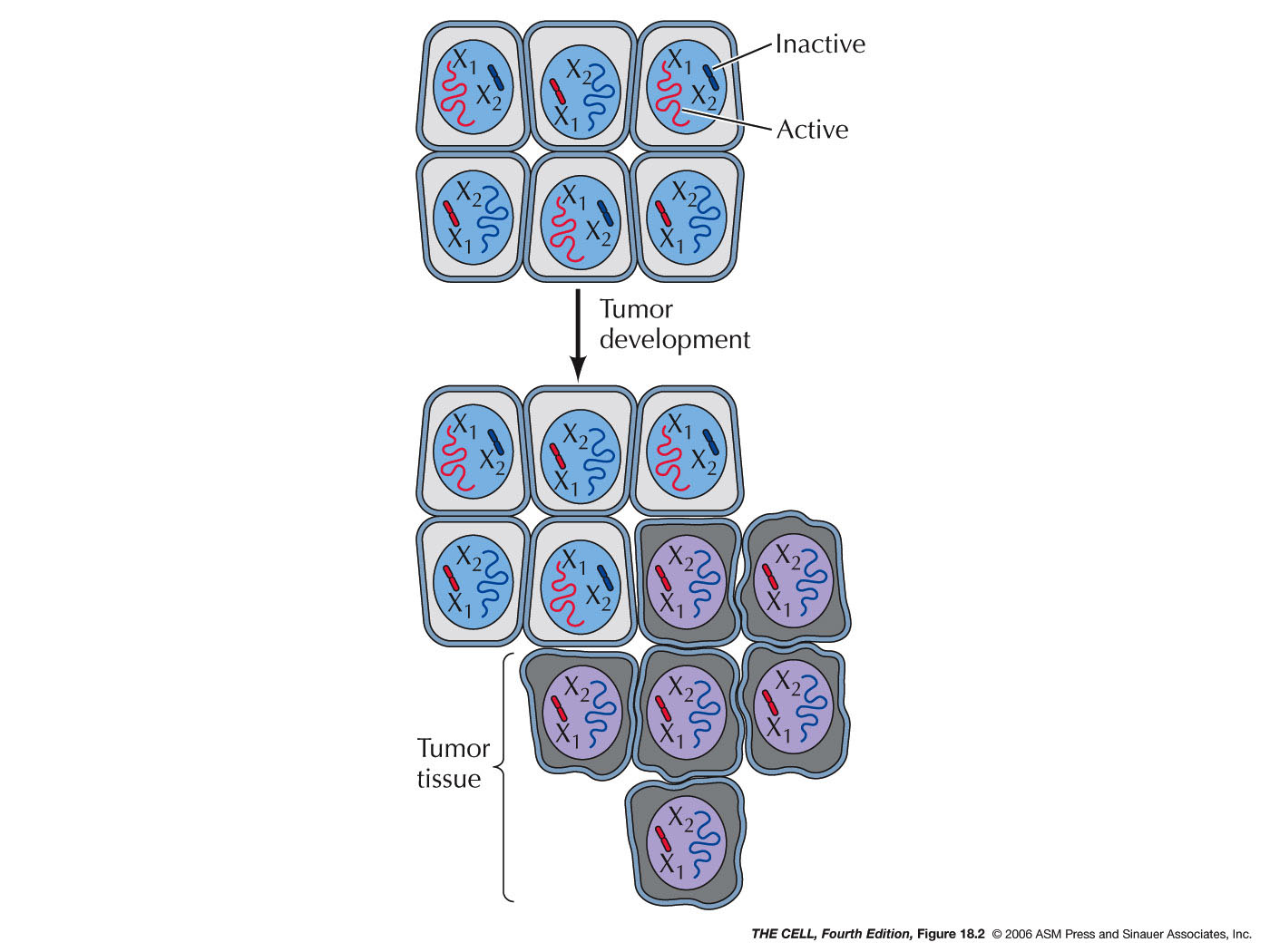
the body.) The clonality of tumors can be shown by
examining the lyonization
pattern
of tumor cells.
- Classification of Cancers: Cancers can
be classified according to their cell-type origin.
- Carcinoma: This is cancer
originating from epithelial cells and represents
about 90% of all cancers.
- Sarcoma: This is cancer originating
from muscular or connective tissue.
- Leukemia and Lymphoma: This is
cancer originating from blood-forming cells or
immune cells.
|
 |
- Characteristics of
Cancer:
- Uncontrolled Cell
Proliferation
- Loss of
Density-Dependent Inhibition: Normal cells
are limited in proliferation by density--availability
of nutrients--and are limited by the availability of
growth factors. Some cancer cells produce their own
growth factors (they show autocrine growth
stimulation).
- Loss of Contact
Inhibition: Contact inhibition is a
characteristic of normal cells. They stop migrating
and dividing when they come in contact with other
cells.
- Lack or Reduction
of Apoptosis (Programmed Cell Death)
- Metastasis:
Production of proteases that digest collagen and other
proteins, together with the the cancer-cell
characteristics listed above, make cancer cell
invasive.
- Angiogenesis:
Tumors secrete growth factors that stimulate blood
vessel growth.
|
- Carcinogenesis:
Mutagenic agents can cause cancer (carcinogenic
radiation, chemical carcinogens, tumor viruses).
- Using Bacteria as Model Organisms:
DNA is DNA, no matter where it is found so a
chemical that is mutagenic to bacterial DNA should
be mutagenic to eukaryotic DNA. Advantages: cheap
and quick. Disadvantages: compared to a chemical
as eaten, many chemicals are modified greatly
before arriving at the DNA.
- Using Mammalian Model Organisms:
If a compound is mutagenic when fed to a mouse, it
is mostly mutagenic to humans. Advantage:
accurate. Disadvantage: Expensive and time
consuming.
- Ames Test--A Good Compromise: A
potential mutagen is treated with a liver extract
then bacteria are exposed to it.
- Carcinogenesis requires multiple
mutational events.
- Genetics of
Cancer: At least two
classes of gene alterations are needed to produce
cancer.
- Oncogenes:
Changes in genes that regulate the proliferation
of cells is a prerequisite to becoming a cancer
cell. An oncogene stimulates the cell to divide in
an unregulated fashion. These genes may enter the
cell by way of a tumor virus, or may become
oncogenes by mutation(s) that occurs to existing
cell-cycle genes (proto-oncogenes)(the latter is
more common). These mutations may be point
mutations, translocations, deletions,
duplications, or gene amplification.
- Tumor Suppressor Genes: In normal
cells, genes are present that would inhibit the
growth of cells containing oncogenes. These are
called tumor suppressor genes. These genes must be
altered or deleted in order for a tumor to become
malignant. (p53 is a tumor suppressor gene that
works in the cells cycle halting division of
abnormal cells, stimulating apoptosis, and/or
stimulating DNA repair.)
- Role of miRNAs:
Increased tumor activity is often associated with
the loss of some normal miRNA activity.
|
- Oncogenesis:
- Oncogenes:
- Viruses:
Some oncogenes are delivered to cells via a virus.
- Proto-oncogenes
and Oncogenes:
80% of human cancer are not induced by viruses
(like some retroviruses), but arise by mutation of
proto-oncogenes to oncogenes. (Retroviruses'
oncogenes are most likely derived from
proto-oncogenes that the virus picked up in a
previous infection.)
- A Specific
Example of an Oncogene: the ras gene family:
The ras
family of oncogenes is the most common oncogene
family in human cancers (25% of all cancers, 50%
of colon carcinomas, 25% of lung carcinomas).
Point mutations convert a ras
proto-oncogene into an oncogene (changing one
important amino acid).
- Normal
ras Gene Activity: Normal
ras
protein (made by the proto-oncogene) is bound
to the cytosolic face of the cell membrane and
may be in a inactive (bound to GDP) or active
state (bound to GTP). When a growth factor,
like platelet-derived growth factor (PDGF) or
epidermal growth factor (EGF), is recognized
by a cell, it binds to a target cell membrane
receptor and the cytosolic face of the
receptor is phosphorylated. This results in
the recruitment of GDP-GTP exchange factor to
the membrane which converts ras
protein into the active (GTP-bound) form. This
sets off a series of reactions that activate
cell division. After this activation has
occurred, the ras protein hydrolyzes its
GTP to GDP and cell division is turned off.
- Mutant
ras Gene: The altered ras
protein (made by the oncogene) is incapable of
hydrolyzing GTP to GDP so cell division is
constituitively turned on.
- Tumor Suppressor
Genes:
- A Specific Example
of a Tumor-Suppressor Gene Altered in Cancer:
the p53 gene: Tumor suppressor
genes inhibit cell proliferation or survival. The
most frequently mutated gene in all cancer is p53,
found in 50% of all cancers.
- Normal p53
Gene Activity: The normal p53
protein controls the cell cycle, DNA repair, and
apoptosis. p53 protein is always made, but
usually is bound to MDM2 protein which degrades
and inactivates it. When DNA is damaged, MDM2
dissociates from p53*, making it more stable and
turning its activity on. (DNA damage stimulates
ATM (a protein kinase) to phosphorylate MDM2 and
to phosphorylate p53, both involved in MDM2
losing its ability to bind to and degrade p53.)
p53's activity is as a transcription factor.
This results in cell-cycle arrest and apoptosis
if the DNA damage is not repaired.
- Mutant p53
Gene: Altered p53 protein found in
tumors cannot arrest the cell cycle, stimulate
DNA repair, and cause apoptosis. This means that
these cells survive and will have higher
mutation rates. (BRCA1 and BRCA2 (common in
breast and ovarian cancers) also cause a similar
ignoring of cell cycle checkpoints.)
- Recent Development in Cancer Biology:
Recent approaches to understanding and treating cancer
include the following.
|
 Home
Home
